Source:
https://rwmalonemd.substack.com/p/lipid-nanoparticles-and-mrna-shots
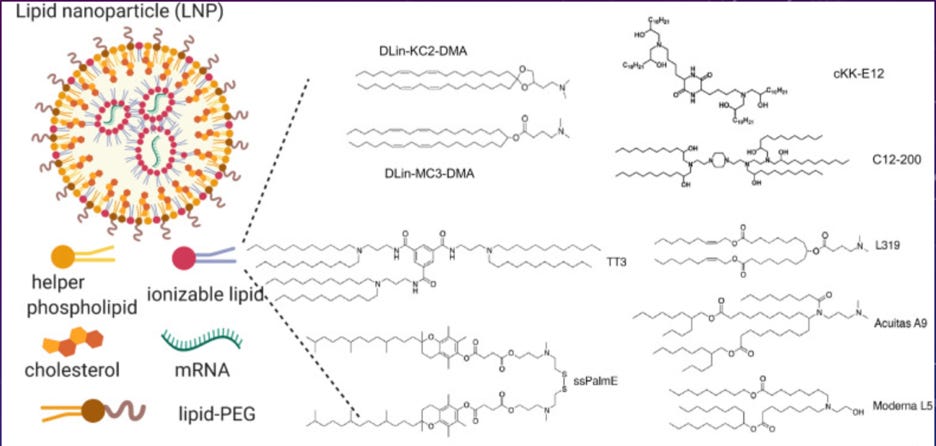
Simplified cartoon diagram of LNP. Upper left image diagrams the complex particle formed by self-assembly of mRNA (a “biological”) and the various chemical (or “drug”) components of the resulting combination product. Lower left provides cartoon diagrammatic images of the individual chemical components. A wide range of ionizable (positively charged) lipids have been developed and tested for use in these formulations, examples of these chemical structures are provided at right. The specific ionizable lipids used vary between the different manufacturers.
Lipid Nanoparticles and mRNA Shots: Did You Take Them Without Knowing What Was in Them?
David Gortler, Robert Malone
In response to the worldwide-spread of COVID-19, a disease caused by Coronavirus SARS-CoV-2, several pseudo-mRNA “gene therapy”-based products were rapidly developed and deployed as prophylactic vaccines. In contrast to recombinant viral “gene therapy”-based vaccines such as those developed by Janssen (J&J) and Astra-Zeneca, these RNA products employ non-viral self-assembling Lipid NanoParticle (LNP) technology to deliver genetic information coding for a viral protein (SARS-CoV-2 Spike) into the cells of the patient. Each of these complicated combination products, which include both a biological component (the RNA) and a complex new ingredient (the LNP), were brought to market by private pharmaceutical companies in a rapidly assembled government-corporate collaboration, operating at “warp speed.”
New vaccine development has historically involved a decade-long discovery, research, testing, review and approval process. In contrast, the detailed and time-consuming methodology required to ensure vaccine product safety and effectiveness were scuttled and the overall process was condensed to less than one year. Next, globally harmonized and previously sacrosanct regulatory and scientific standards, carefully developed over decades were discarded when it came to the Emergency Use Authorization (EUA) for the completely novel and barely tested mRNA-based injections.
Although intended to prevent viral infection, replication, spread and COVID disease or death, and to enable “herd immunity”, these products were developed at “Warp Speed” and labeled “vaccines” but differed remarkably from all other currently available vaccine products. That haste and the associated regulatory compromises enabled by lax EUA requirements yielded products with high rates of avoidable existing and emerging treatment-associated serious adverse events including hospitalizations, permanent disabilities and deaths, as reported in both VAERS, and the CDC’s now surreptitiously shuttered V-safe reporting system. The abbreviated testing associated with this novel mRNA/LNP combination product should have led to a substantially greater contemplative pause, well-prior to authorizations and federal implementation and vaccine mandates from federal officials within the executive branch of government.
Damn the Torpedoes
It was obvious to those who were familiar with the scientific method and investigational medicine what could – and ultimately did occur. Drug development processes have a very high failure rate, and the number one reason for clinical failure of investigational medicines is drug safety. Despite that, the government discarded established norms for testing and development with these products. This policy has resulted in avoidable damage to both public trust in government and regulatory authority integrity. Policies and practices based on decades of “lessons learned” from prior product development failures were jettisoned with little discussion or justification.
Going forward, a central unresolved issue is whether federal regulatory authorities will accept responsibility for oversight failures during the COVID crisis, and recommit to fulfilling their essential role in ensuring the safety, purity, effectiveness and consistency of drug and biological medicines administered to their citizens.
The terminology used to define and describe this subcategory of non-viral gene (polynucleotide) delivery formulations can seem very intimidating. For this discussion, we will focus on just introducing the components, their general characteristics and biochemistry, and not on the pseudouridine-incorporating “mRNA” payload which these particles deliver into cells.
“Star Trek” Science Fiction and Vaccine Development?
Those who are not familiar with the underlying formulation chemistry behind this technology employed by the whole of government “Warp Speed” project (a reference to the “Star Trek” science fiction series) often reach for other Star Trek metaphors, including nanites or programmable nanobots, but the formulations and chemistry has nothing to do with nanites or nanobots.
Described and introduced in the first episode of the third season of “Star Trek: The Next Generation” (titled Evolution) nanites are imagined to be microscopic robotic devices built by manipulating atoms, capable of mechanical self-replication, and containing gigabytes of computer memory. In this science fiction script, they are used by the Federation for medical purposes and are designed to work inside nuclei during cellular surgery. When not in use, nanites are stored in a non-functional state and can be destroyed with a burst of high-level gamma radiation. The Borg Collective used nanites on their Diamond vessels as a method of disrupting enemy communication and computer systems. While not weird or strange in the imaginary 24th century universe of Star Trek, nanites or nanobots are a figment of people’s imagination in today’s 21st century.
Acknowledging that the CIA’s Defense Advanced Research Project Agency (DARPA) and the Department of Defense often reach to science fiction speculation when setting goals and objectives for development of new warfare technologies (for example neural implants, fighting exoskeletons and other forms of transhumanism), the formulation chemistry employed in the mRNA vaccines is crude and mundane by comparison to the imaginary “Borg Collective” technology used in an imagined 24th century “Star Trek” reality. Those using the terms “nannite” or “nanobot” for the current pseudo-mRNA based COVID vaccine products are either confusing science fiction with current reality, or are unscrupulously trying to scare the public by using threatening terminology from a fantasy future universe. Whatever the cause or intention, use of these terms in alternative and social media is yet another example of fearporn.
Clinical Nanotechnology is Novel and Experimental
What is nanotechnology? Besides being a hot buzzword which can greatly increase interest and funding for a new technology (or intimidate the uninitiated), according to the European Director General for health and consumer protection, “nanotechnology refers to the branch of science and engineering devoted to designing, producing, and using structures, devices, and systems by manipulating atoms and molecules at nanoscale, i.e. having one or more dimensions of the order of 100 nanometers (100 millionth of a millimeter) or less. In the natural world, there are many examples of structures with one or more nanometer dimensions, and many technologies have incidentally involved such nanostructures for many years, but only recently has it been possible to do it intentionally. Many of the applications of nanotechnology involve new materials that have very different properties and new effects compared to the same materials made at larger sizes. This is due to the very high surface to volume ratio of nanoparticles compared to larger particles, and to effects that appear at that small scale but are not observed at larger scales.”
Are the ”self-assembling lipid nanoparticles” used in the RNA delivery technology employed by BioNTech/Pfizer and Moderna actually nanotechnology? Well, technically they are not. These formulations are associated with a wide range of particle sizes which typically vary from 100+ nanometers to a micron or more in size. Furthermore, once formulated, the particles have a tendency to form into even larger aggregates that are often toxic but not functional for RNA delivery purposes. This is the reason which the formulations developed at the University of British Columbia by Dr. Pieter Cullis and colleagues, which are the “enabling” technical advances for the current generation of “(pseudo)mRNA” vaccines, all include added polyethylene glycol (PEG) to reduce aggregation while stored before injection.
LNPs are Highly Complex mRNA Delivery Vehicles with a Questionable Track Record
Most people will see the term “lipid nanoparticles” and maybe think: okay, those are just lipids, but smaller. As will be explained, there is a lot more to it. Avoiding technical nuances, LNPs are aggregates of both positively charged chemicals and negatively charged RNA (RNA and DNA are two types of “polynucleotides”). You can think of RNA as being like a long single-stranded chain of pearls. The string connecting each pearl is a chemical structure which has a negative charge, so a string with lots of pearls (nucleotide bases) will have lots of negative charges. The positively charged chemicals are synthetic fats, otherwise called lipids. Opposites attract, and so when RNA and positively charged fats are mixed in water-based solutions, they find each other (“electrostatic attraction”) and the chemically positive part of the fats will loosely attach to the negatively charged parts of the RNA. The result is a “self-assembling particle” (because no real tricks are needed to make these come together), which forms a sort of blob of mixed fats coating one or more RNA molecules.
Of course, the details matter; which chemically synthesized LNPs work best for binding the RNA, then binding and fusing with cells, and then releasing the RNA is more or less an art form, or really a guessing game. Guessing games and plausibility as a substitute for hard, long-term clinical safety and efficacy findings aren’t a good idea when it comes to investigational medicine or clinical pharmacology under any circumstances. It’s especially the case when they are distributed to billions of unique individuals worldwide.
LNP Variability Factors and Potential Clinical Manifestations
There are few options to control the spontaneous lipid “self-assembly” process, leading to product consistency issues. The concentrations and mixing process can be controlled to some extent, and technologies for mixing and shearing particles such as sonication and microfluidization (pushing the resulting liquid mixtures through a tiny hole) can influence the resulting average particle composition and size. But no matter what is done, after “formulation” the resulting inherently heterogenous mixtures begin as a range of sizes and they typically change over time – particularly when storage conditions are varied.
Product consistency is critical when it comes to clinical application of any product – let alone a completely novel, rapidly developed, biotechnologically complex one that is highly dependent on environmental conditions such as pH and temperature. Product consistency is one of the many things that should be monitored and shared by the FDA’s Office of Clinical Pharmacology (and its 1,300 employees) but Americans have zero transparency on any of those quality control findings. In addition to the inherent differences in LNP manufacturing and mRNA quantity (dose), is the time between controlled temperature storage and injection into a patient. Controlling that variability in regimented clinical trials -- where every aspect is militantly structured via a protocol -- is easy; broad circumstances in the real-world involving billions of doses plus boosters in an atmosphere of promoted unjustified panic, is a much different scenario.
Different FDA Regulatory Standards for Different Products
From a regulatory and testing standpoint, these “vaccines” are extremely complex products. Typically, the world of regulated pharmaceuticals is divided into drugs, devices, and biologicals. A new drug is usually a “new chemical entity” which can be synthesized or purified as a single chemical, and readily characterized for purity, identity (is the chemical what is advertised?), adulteration (whether or not there are incorrect or altered ingredients), and activity (does it work as intended?) using standard, well-developed methods. Devices are things like pacemakers or diabetic glucose meters, and have a variety of physical and performance characteristics that can also be easily tested.
Biologicals (including RNA, DNA, proteins and recombinant viruses) are a newer category of pharmaceuticals. Generally speaking, if analyzed like a drug, two different biologicals can have identical chemical composition, purity, etc. and still act very differently. This is because biologicals (usually proteins but also nucleic acids) are typically so large that the way that they fold and bend can have a substantial impact on their pharmaceutical activity. And this bending, folding, and other properties are directly affected by small details of how they are manufactured, purified, formulated, stored and administered.
Next are combination products, or those that are made by combining drugs/ devices/ biologicals. The RNA products intended as COVID vaccines are actually very complicated combination products; they combine new synthetically engineered nanochemicals that have not been previously tested and characterized in a large cohort of individuals for an extended period of time, chemicals which are known to the FDA (such as polyethylene glycol or cholesterol), RNA biologicals, and relatively unpredictable and non-homogeneous self-assembling components. These “cationic lipid” (positively charged lipids) -based polynucleotide delivery products are a very specialized subcategory of pharmaceuticals, and there are very few true “experts” in this area – and it is unknown if there is relevant expertise within the FDA. These pharmaceuticals have no natural analogs.
It is possible that these positively charged, synthetically-manufactured lipids, which do not appear to exist naturally should be considered a novel biotechnology all by themselves, with independent safety/toxicological profiles based on their configurations.
Chemistry 101: Electrical Charge as a Method of Cell Targeting
So how do these complicated but relatively crude particles work to deliver RNA or DNA molecules into cells? The truth is that this is not completely understood. The key discovery was sort of happenstance, as is often the case with scientific breakthroughs.
During the 1980s, a pharmaceutical formulation scientist working at Syntex (in the California bay area) was working with liposomes (which are sort of like sub-microscopic ziplock bags made of synthetically engineered lipids that can be filled with drugs or other things). He was researching ways to change the charge on nerve cell outer membranes, which are generally negatively charged as are virtually all animal cells. He (and other researchers before him) had a chemist manufacture lipids that would participate in forming liposomes but were positively charged (something that doesn’t occur naturally). When mixed with other lipids that would fuse with cell membranes to form liposomes, these could then be applied to the negatively charged nerve cells. Since opposites attract, these particular liposomes would electrically bind and fuse to the nerve cell membranes.
Many experimental cell culture studies performed over the decades since initial discovery suggest that these cationic lipids could be responsible for vascular damage, stroke or other toxicities associated with adverse events shown through various adverse event reporting databases. For example, published safety data sheets clearly state that the cationic lipids SM-102 and ALC-0315 are not for use in humans.
A student that had worked with bacterial DNA (plasmids) at a leading recombinant DNA laboratory at UC Davis (Bolivar and Rodriguez – who created the plasmid pBR322) was invited to intern in the nerve cell/liposome lab. She had the bright idea that DNA was negative, and these liposomes that fused with cell membranes were positive. What happens if they are mixed together? Could the DNA get into the cells during the fusion process? And thus a whole new field of pharmacology was born.
To understand what appears to happen requires a bit of modern cell biology. Cells are defined by a double layer of fats which form a membrane on the outside of the cell. Both the inside and the near outside of every cell is surrounded by water, but not the liquid water that most of us are used to. Water has four chemical forms; liquid, solid (ice), gas (steam), and a fourth form which is a type of gel, or structured semi-fluid. Any who have looked into what keeps the free water locked into a modern disposable diaper is familiar with the gel form of water. Cells (and viruses) use chains of carbohydrates that stick out of their membranes or are attached to membrane proteins to control the structure of the water surrounding them. This can make it very hard for things to get close enough to a cell membrane to mix or “fuse” with the cell membrane. Particularly big things, larger than one micron. In contrast, really small, truly nanoscale particles (or small individual chemicals) can often cross membranes relatively freely. Unless something punches or tears a hole or otherwise disrupts a cell membrane, large, highly charged polymers like DNA or RNA cannot easily get across cell membranes -- which is a good thing, or else we would never be able to develop as separate species. Remember that cells are generally negative and so are DNA and RNA, so they repel each other, making the situation even more challenging for those who which to introduce foreign polynucleotides into cells. This set of issues defines the delivery problem which must be solved for any non-viral gene therapy technology.
One way to solve this “delivery” problem is to make a particle of collapsed or condensed DNA or RNA by coating it with positively charged lipids, which are mixed with other lipids that will easily mix with cell membrane lipids. And to work well, the particles must be small enough (ie, nano) that the attractive force of their positive charge is strong enough to force them through the structured water around a cell so that they can get so close that their lipids will mix with the cell membrane lipids, and somehow this either brings the DNA or RNA into the inside of a cell or into “endosomes” which are sort of like big liposomes within the cell, and then the DNA or RNA gets released from there.
The other main problem is that the fluid in one’s blood, lymph and surrounding your cells is full of other charged molecules (mostly proteins) that can also bind the RNA or DNA/lipid complexes, and block them from getting close to the cell membrane. And that problem seems to have been at least partially solved by Dr. Pieter Cullis and his colleagues at the University of British Columbia with their advanced, PEG-containing formulations and special synthetic (a new biological entity?) positively charged lipids.
Most Basic FDA Structure/Dosage Information Lacking
While basic mechanism and formulation information is known, to this day many technical details are lacking (including the most basic details). For example, the actual the dose/number of mRNA strands per injection hasn’t been provided by manufacturers or the FDA. The molecular mass can’t even be estimated mathematically on the basis of Avogadro’s constant, because neither the actual mRNA sequence nor its mass are specified in manufacturers’ package inserts. Pharmacists know to administer a 0.3 ml dose; but exactly what quantity of mRNA/LNP is in that volume of fluid? Just as so many other well-established regulatory norms were discarded, the fundamental bioethical and clinical mandate for patient informed consent prior to administration of any pharmaceutical or procedure was arbitrarily and capriciously disbanded by both government regulators and pharmaceutical industry sponsors in the name of a Public Health Emergency.
On top of that, package labeling that was released many months later (p.20) only states: “Each 0.3 mL dose … contains 30 mcg of a nucleoside-modified messenger RNA (mRNA) encoding the viral spike (S) glycoprotein of SARS-CoV-2” …never specifying the sequence. The label goes on to state: “…nucleoside-modified mRNA in COMIRNATY is formulated in lipid particles, which enable delivery of the mRNA into host cells to allow expression of the SARS-CoV-2 S antigen.” The label then lists, glycols, sterols and cholesterol, but lipid nanotechnology and LNP configuration(s) are never mentioned. It is akin to describing a drug’s structure as containing “carbon, oxygen and hydrogen” without ever showing the actual structure. As a comparison, manufacturer package inserts typically contain a graphic of the chemical structure of their product – even when they are widely known.
In other words, more than three years later, with well over half a billion shots being given in the USA alone, there is still much perfunctory information regarding not just the configuration and potentially dangerous clinical manifestations of these shots, but the actual dosage and ingredients themselves.
Lawsuit for Transparency
In fact, a lawsuit demanding transparency had to be filed by attorney Aaron Siri to compel the FDA to release one manufacturer’s application, including mRNA sequence(s) and LNP configurations. One manufacturer’s mRNA application to the FDA is said to be ~1.2 million pages long. In response to consumer questions, the FDA initially very dubiously proposed redacting and releasing only 500 pages per month which would have taken ~200 years, which is more than a little ironic seeing as how the entire “warp speed” development and EUA process took less than one year.
The judge ordered the FDA to release 55,000 redacted pages every 30 days, which will still take two years. While that may seem like a relative bargain -- it really isn’t. Any FDA medical officer/senior medical analyst (present author included) can tell you that the vast majority of that application is nothing but raw tabled numbers (individual subject lab values, et cetera) that requires little/no redaction since no names or other PHI or HIPAA information are included in unredacted applications. Additionally, since the judge’s order didn’t specify that any particular pages would be given priority or should be released sequentially, the most critical parts of the application, such as LNP safety, LNP configurations, mRNA LNP binding sites, the actual mRNA strands or milligram/LNP quantity and sequence per 0.3mL injection could be the last pages to be released. That prolongs the delay of the most fundamental information needed by outside drug safety analysts, clinicians and other scientists for modeling potential mechanisms for the established safety signals.
Fully Taxpayer-Funded, but LNP Safety and mRNA Sequence(s) Still “Trade Secret”
There is no good reason why Americans do not have full disclosure on mRNA sequence, LNP configurations and toxicity studies. Manufacturers have total liability immunity under the Public Readiness and Emergency Preparedness Act (PREP Act). Indeed, one manufacturer alone acquired an unprecedented $100 Billion profit -- in just one year – $38 Billion of which was directly from mRNA shots, not counting other taxpayer-provided, Covid-related “warp speed” funding.
Nevertheless, all manufacturers claim critical information about these products to be “trade secrets.” This is atypical; all other FDA approved products – even older ones such as amoxicillin prominently detail all of their ingredients (including the full ingredient list plus active ingredient quantity and specification of whether ingredients are either active or inactive components) of their product within their official product labeling.
The current labeling additionally doesn’t specify pharmacologically/toxicologically active versus inactive ingredients, and genotoxicity and carcinogenicity studies have yet to be done. To illustrate one clinically significant consequence of this failure to disclose, a substantial number of patients have pre-existing allergies to PEG. These patients are at risk of anaphylactic shock, one of the most common immediate short term adverse events associated with injection of these products. Patients should have been informed of the presence of PEG in the formulations, and those with known history of PEG anaphylaxis should not have received the COVID mRNA vaccines.
LNPs and Human Safety/Toxicity
LNPs, being a new and largely untested nano-biotechnology, have both generally and specifically come into question regarding their track records of toxicity and safety. Because they are considered engineered “nanotechnology”, they are not necessarily just smaller versions of existing or naturally occurring lipids. As stated earlier, LNPs and positively charged lipids do not occur naturally, and their self-assembly is not a well-controlled process. In addition, as per the design, ‘disassembly’ or content dumping is also not a well-controlled process. Unfortunately, the FDA does not appear to have kept up with its safety mission, regulating or fully testing LNPs as a novel and emerging technology, for which consistency, individual safety or cell targeting has not been fully elucidated.
In vivo studies for the safety of LNPs prior to their release under EUA have seemingly neglected to examine the potential standalone safety of LNPs themselves, instead they were inferred to be an inactive, inert “vehicle” of mRNA injections. However, these LNPs, depending on their size and other factors, are not simply inactive transporters. Purification, charge, substrates and size-based separation of nanoparticles are some of the challenges, that can affect LNP cell-targeting activity.
LNP Standalone Safety/Toxicity in mRNA Shots Have Never Been FDA Tested
We don’t know if the FDA required discrete safety tests based on LNP characterization. In fact, we don’t know whether the FDA or manufacturers required any LNP safety or toxicology studies on this LNP nanotechnology at all. LNPs, being positively charged, (lipids are usually neutral or negative) would likely target negatively charged human cells, separate from any other LNP-substrate-targeting abilities. Much like the numerous variations in carbon-based structures of small-molecule pharmaceuticals, the possibilities of LNP configurations and/or their substrate attachments have the potential to be extensive, and could also give them the ability to specifically target individual cell receptors.
Un-Regulated Biotechnology? Official “FDA Guidance Documents” on Both Liposomes -and- Nanotechnology Exclude any Mention of LNPs
There appears to be a “regulatory vacuum” in FDA guidance when it comes to LNPs, excluding them from guidance oversight and specific FDA safety testing recommendations.
Firstly, the FDA’s guidance document recommendations on Liposome Drug Products considers liposomes to be “…vesicles composed of a bilayer and/or a concentric series of multiple bilayers” (emphases added). While liposomes may be considered a type of LNP, LNPs are known to only have a lipid monolayer, thereby excluding LNPs from that guidance.
Additionally, in the FDA’s Guidance for Industry: “Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology” does not mention “lipids” anywhere within its text. While lipids are a common component in multiple pharmacological preparations, this seems to be the first market authorized vaccine product with bioengineered lipid nanoparticles.
It should have been obvious to mRNA manufacturers and/or the FDA that both LNPs and liposomes can be highly and similarly programmable to target specific cell receptors, and that LNPs should have been subject to special attention and testing for safety prior to their extensive implementation. Yet, it remains unknown if any such safety tests occurred during “warp speed” development, followed by the aforementioned profanely lucrative mRNA injection rollout.
“Safe and Effective?” LNPs and Spike Proteins and Life-Threatening Inflammatory-Related Adverse Events
Several studies have shown that spike proteins from either the mRNA shots or infection are toxic in a dose-dependent manner. The mRNA injections can turn one’s own cells into manufacturing machines compulsively replicating just the toxic engineered SARS-CoV-2 spike protein at a potentially greater rate than what would be via a community acquired COVID infection. While a healthy immune system will build antibodies and fight SARS-CoV-2 viral particles, attenuating replication, an mRNA injection has the potential to produce more coronavirus spike proteins, and at a higher rate, depending on the number/load of mRNA strands in the “vaccine” injection. As of now, we still don’t have transparency on the number of mRNA strands in a single injection or lot-to-lot variability.
In addition to the independent toxicity of spike proteins, there is a history showing that certain LNPs by themselves have independent toxicity and are known to activate the complement (inflammatory) system. In fact, Dr. Robert Malone, the inventor of mRNA delivery in animal models has written about how he was never able to overcome toxicity of any lipid-based delivery mRNA or DNA, eventually shuttering lipids as a delivery vehicle – and that was back in the 1990s. Was the FDA not aware of that history?
Today, published studies detail the inflammatory-centric adverse events seen with mRNA injections including ischemic stroke, pericarditis and/or myocarditis. Pathological inflammation is at the very core of many other adverse events reported in the FDA’s VAERS and CDC’s V-Safe reporting systems, which is clearly indicative of host immune responses against transfected cells signaling for termination via cytotoxic T cell killing, for example.
On top of published studies, mRNA injections have been reported to FDA’s VAERS as the primary adverse event suspect in >20,000 heart attacks and >27,000 cases of myocarditis and pericarditis as reported in the USA alone. Even worse: according to over a dozen published studies, (including a recent FDA-funded study out of Harvard) the adverse event numbers reported in FDA’s VAERS represent fewer than 1% of vaccine adverse events that actually occur in the USA.
In sum, LNPs have the potential to function as much more than a simple lipid (in fact, they are clearly biologically active drugs), and may have independent uncharacterized clinical or safety/toxicity effects. Detailed descriptions of specific cell-targeting specificity, affinity, toxicology and safety of these LNPs ought to have been conducted and required as part of the review and approval process by the FDA -- but it doesn’t appear that they were.
In an exuberant rush to roll out mRNA shots, did LNP regulation fall through a “regulatory crack” at the FDA? Are the potential variabilities in LNP component(s) of these mRNA injections responsible for the serious, and inconsistent adverse events seen with these injections? These important questions cannot be answered until we are enlightened with a lot more specificity regarding ingredient consistency of the COVID vaccines, plus a detailed description and independent safety studies of the specific LNPs employed.
It appears America’s drug safety experts will simply have to wait another year or so for the FDA to comply with the court’s disclosure order for a list of mRNA vaccine ingredients including detailed descriptions of LNPs. We can only hope that when the FDA finally does fulfill its obligation, that it doesn’t absurdly over-redact its documents to the point of being incomprehensible, as it has done in the past (see sample redactions in preceding link).
In the meantime, there are important transparency issues and legitimate, unanswered questions about mRNA formulations and safety, despite the harmonized “safe and effective” propaganda chorus from federal officials, vaccine industry shills and corporate media concerning the approved fall 2023 “booster” injections.
(Author David Gortler has not reviewed the final version of this text)

No comments:
Post a Comment
Note: only a member of this blog may post a comment.